Tired of ACNU & SaMD Launches
Derailed by Complexity?
You know the potential of OTC switches and digital therapeutics. You also know the hurdles:
Vendor Sprawl
Juggling separate app devs, QMS tools, validation consultants, and retail integrators.
Regulatory Paralysis
Navigating the maze of ACNU rules, SaMD validation (IEC 62304), and 21 CFR Part 11 is slowing you down.
Integration Nightmares
Connecting digital screening to the point-of-sale feels like plumbing the impossible.
Compliance Risk
Audit trails, failure reporting, documentation – one slip means costly delays or worse.
Stop stitching together solutions.Start launching with structure.
Everything You Need to Go OTC,
Digitally, and Compliantly.
AEGIS brings ACNU and SaMD execution into one platform — from pilot to post-market.
ACNU Screening Engine
Build and deploy FDA-ready self-screening workflows with branching logic, EHR integration, and automated validation.
SaMD-Ready Framework
IEC 62304 and ISO 13485 compliant lifecycle support with version control and traceability for digital health submissions.
Automated Compliance
21 CFR Part 11 compliant records, automated ACNU failure reporting, and FDA-ready audit trails built-in.
E-Commerce Integration
Verification API for seamless POS and e-commerce integration. Connect eligibility to purchase instantly.
Real-Time Analytics
Monitor screening outcomes, success rates, and post-market safety signals with comprehensive dashboards.
Content Management
Centralized CMS for educational content, digital labeling, and multi-product program management with version control.
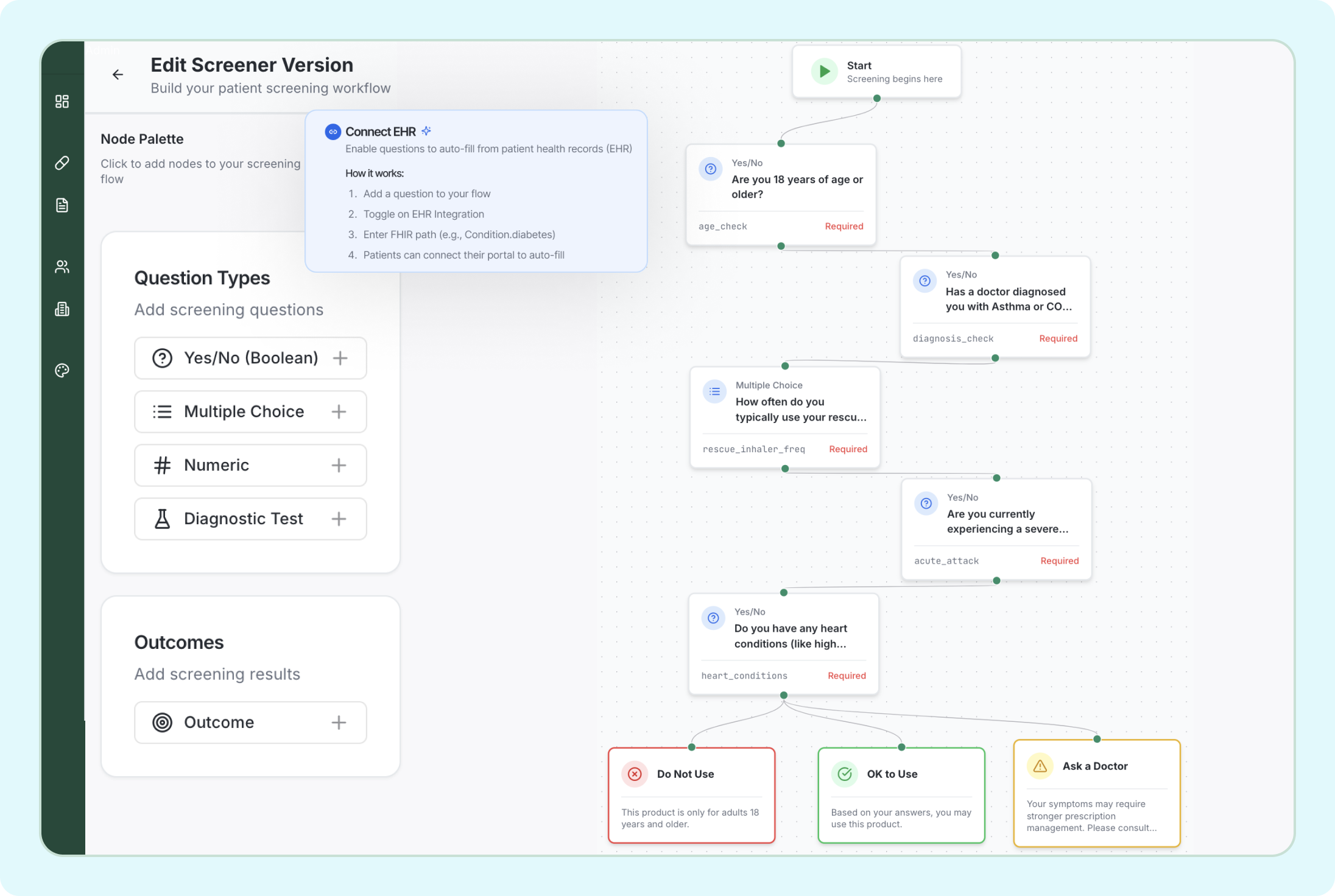
Build FDA-Ready Screening Workflows
Visual workflow builder with drag-and-drop nodes, EHR integration, and automated compliance checks. Create complex ACNU screening logic without writing code.
- Drag-and-drop question nodes
- Connect EHR for auto-fill
- Built-in validation rules
- Real-time compliance checks
Seamless Screening,
From Question to Clearance
AEGIS delivers a patient-friendly screening experience that's compliant, secure, and conversion-optimized.
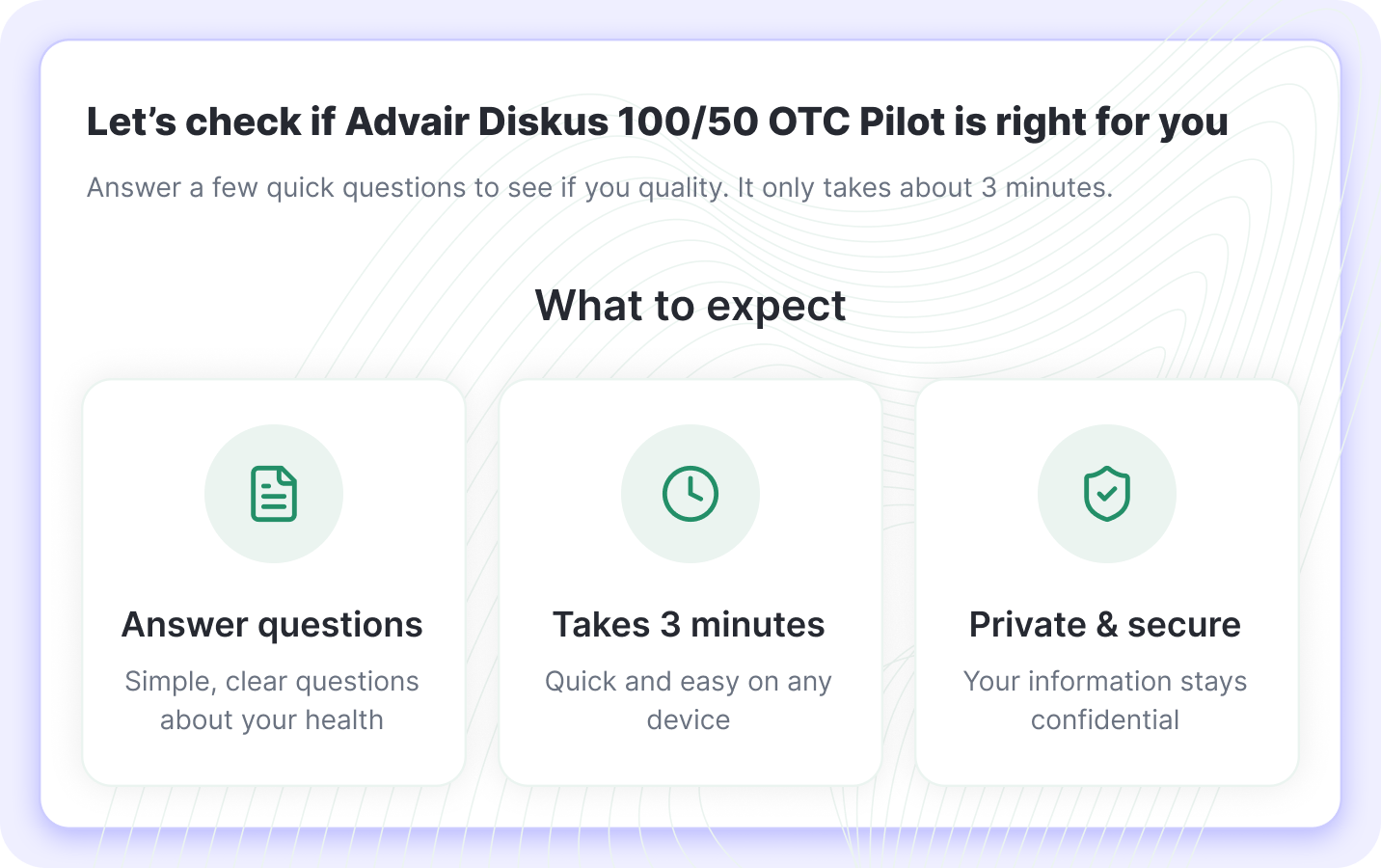
Clear Introduction
Patients see what to expect: simple questions, 3-minute completion, and privacy assurance.
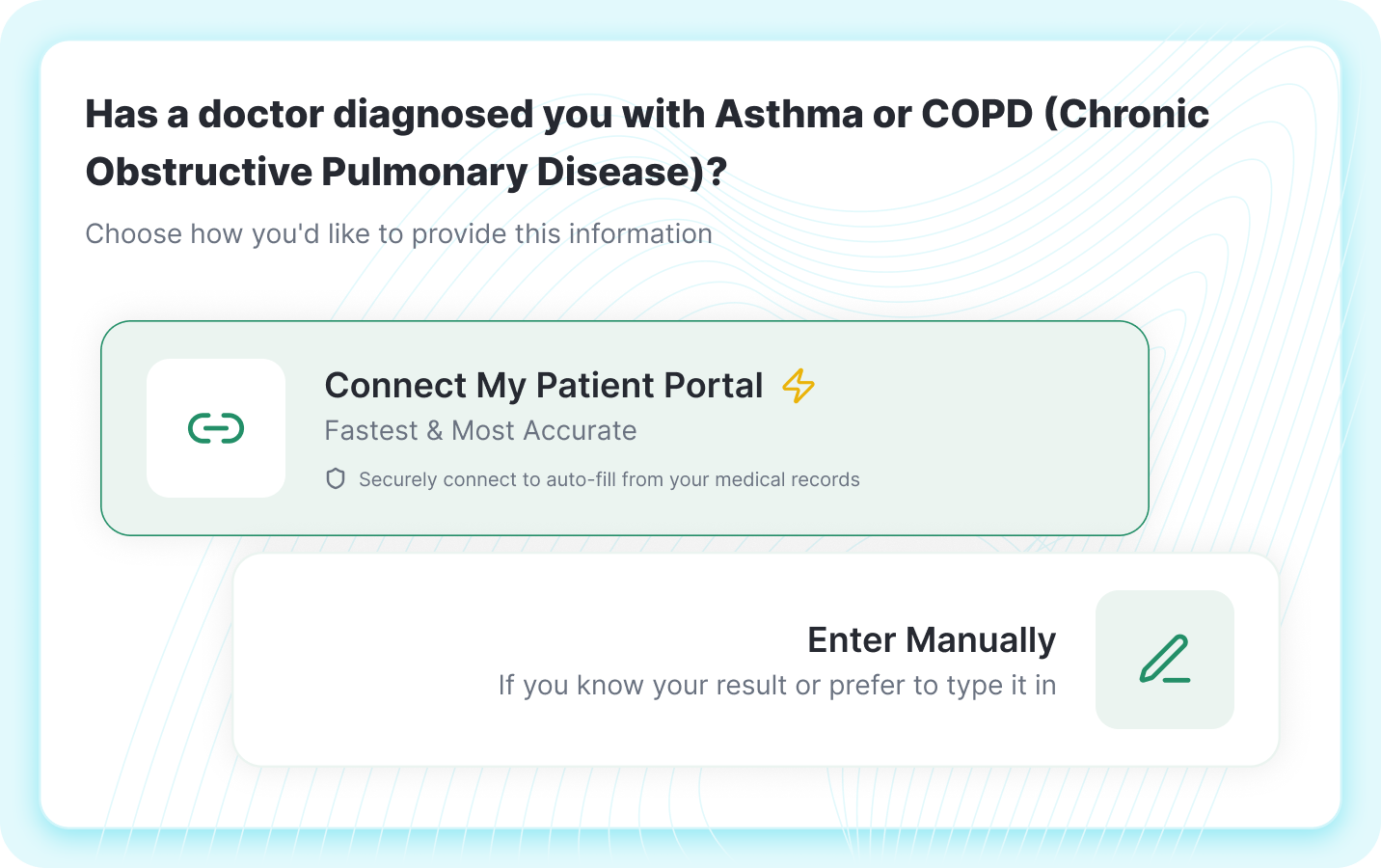
Smart Screening
EHR integration allows auto-fill from patient portals, or manual entry for flexibility.
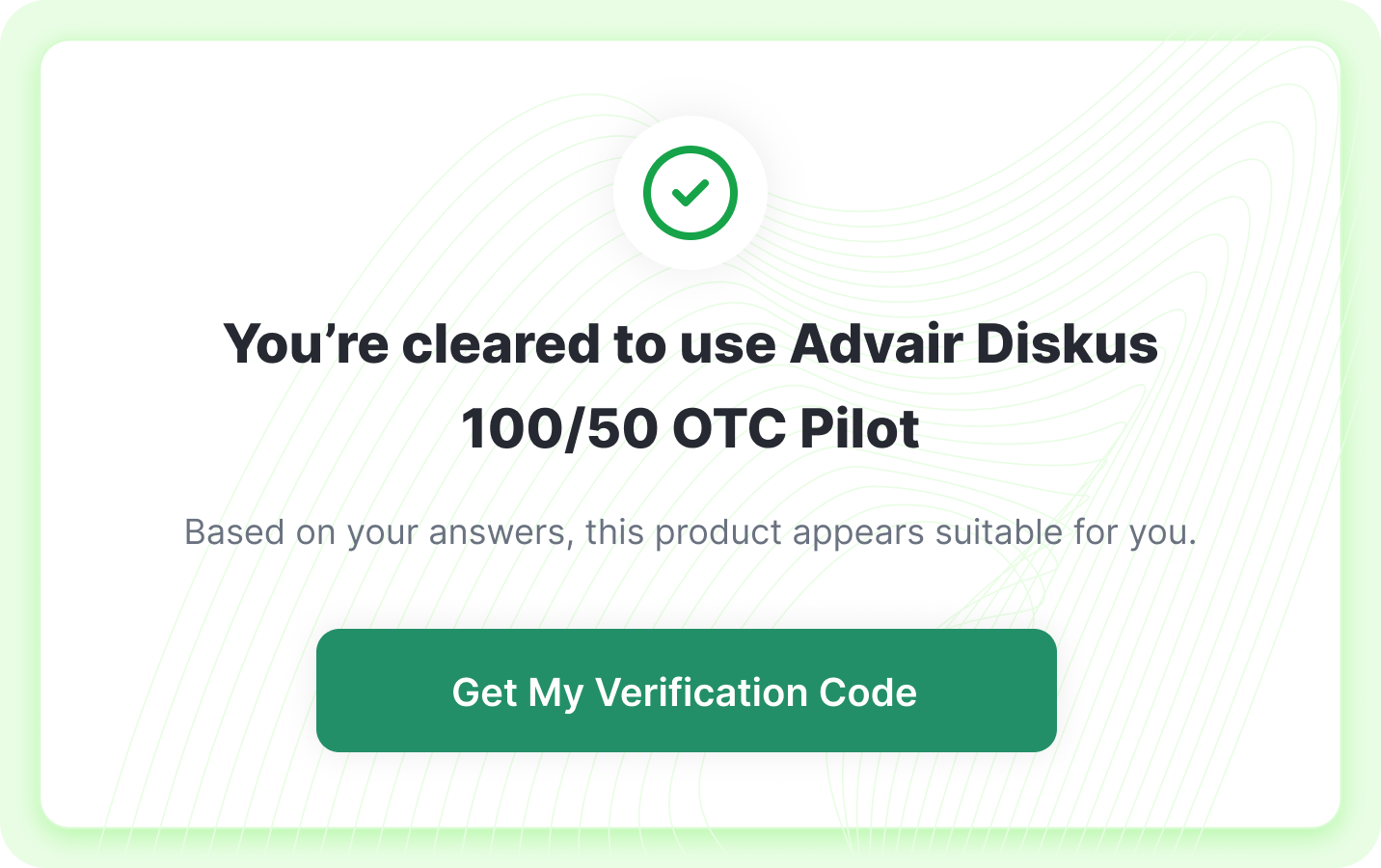
Instant Clearance
Patients receive immediate eligibility results with next steps to purchase or consult.
Beyond Screening:
Complete Patient Support
AEGIS powers comprehensive patient engagement from initial screening through ongoing medication management and support.

ACNU Screening
FDA-compliant safety questionnaires in-app
Patient Education
Videos, safety info & digital labeling
Smart Reminders
Push notifications for adherence
Easy Refills
One-tap re-ordering & subscriptions
Seamless Payments
HSA/FSA & insurance integration
Live Support
Instant chat & telehealth access
Enhanced Patient Support
& Clinical Trial Workflows
AEGIS provides comprehensive patient support and flexible clinical study capabilities through configuration, with continuous platform evolution.
Core Capabilities
Patient Support
Comprehensive support infrastructure with embedded help and educational content throughout the screening experience.
- Embedded support links in screening flows
- Contact information for patient inquiries
- Educational content delivery via CMS
- Digital labeling and safety information
Clinical Study Support
Configuration-based study capabilities for pilot programs, actual-use studies, and clinical trials.
- Draft screeners for study cohorts
- QR code generation for study enrollment
- Screening session data analysis
- Study-specific configuration options
- Separate study and commercial environments
Continuous Platform Evolution
AEGIS continuously evolves to support advanced patient engagement and clinical trial workflows. Our roadmap includes:
Advanced Patient Support
- •Medication adherence tracking and reminders
- •Automated refill notifications and management
- •Integrated telehealth and live chat support
- •Pharmacist consultation scheduling
Dedicated Study Mode
- •Study-specific flags and participant tracking
- •Unique participant ID management
- •Study-specific surveys and assessments
- •Advanced cohort analysis and reporting
Configuration-First Approach
AEGIS supports clinical studies through flexible configuration rather than dedicated in-app features. This approach allows sponsors to conduct pilot programs, actual-use studies, and label-comprehension trials using the same compliant infrastructure that powers commercial ACNU programs—without waiting for specialized study modules.
Launch ACNU & SaMD Programs
Months Faster, With Less Risk.
AEGIS eliminates the friction between digital development, regulatory affairs, and commercial teams.
Accelerate Timelines
Cut validation and integration time significantly. Go from pilot to launch faster.
De-Risk Your Submission
Build on a foundation designed for FDA scrutiny from day one.
Unify Your Stack
Replace multiple vendors with one integrated, compliant platform.
Focus Your Team
Let your experts concentrate on clinical strategy, not software infrastructure.
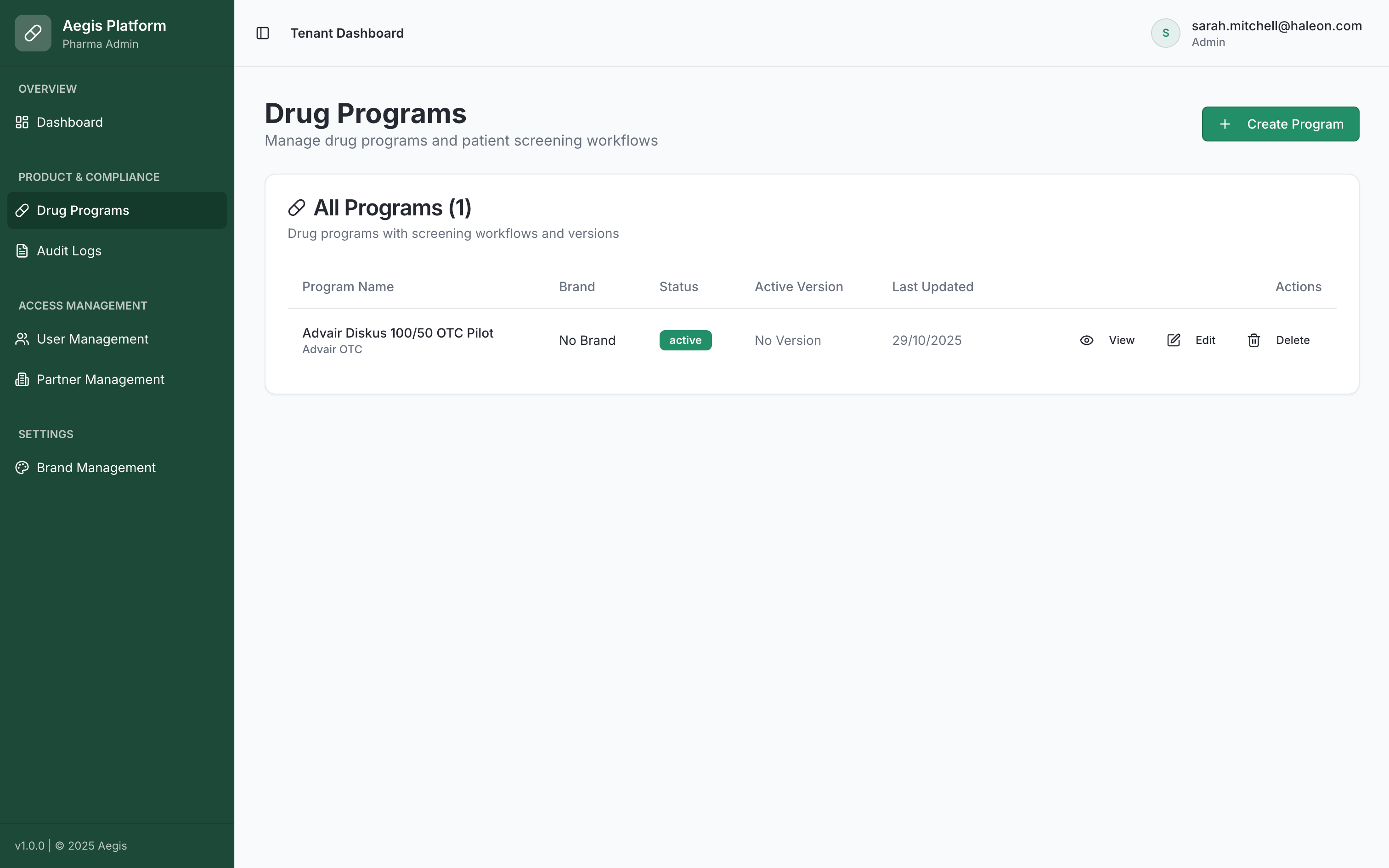
Centralized Program Management
Manage all your drug programs, screening workflows, and compliance documentation from one unified dashboard.
- Multi-program oversight
- Version control & audit trails
- User & partner management
- Real-time compliance monitoring
Audit-Ready,
Always.
AEGIS is engineered for the highest regulatory standards with comprehensive certifications and compliance frameworks.
ACNU-Ready
Configurable screeners, automated failure reporting
SaMD-Ready
IEC 62304 / ISO 13485 compliant lifecycle support
Data Integrity
21 CFR Part 11 / Annex 11 compliant records & audit trails
Secure & Scalable
SOC 2 Type II, ISO 27001, HIPAA-aligned infrastructure
Long-Term Compliance
10-year immutable record retention
Global Standards
MDSAP, GDPR, and international regulatory alignment
Industry Certifications





Everything You Need to Know
About AEGIS
Get answers to common questions about AEGIS, ACNU, SaMD, and regulatory compliance.